Research Highlights
UNDERSTANDING VIRAL PROTEASES
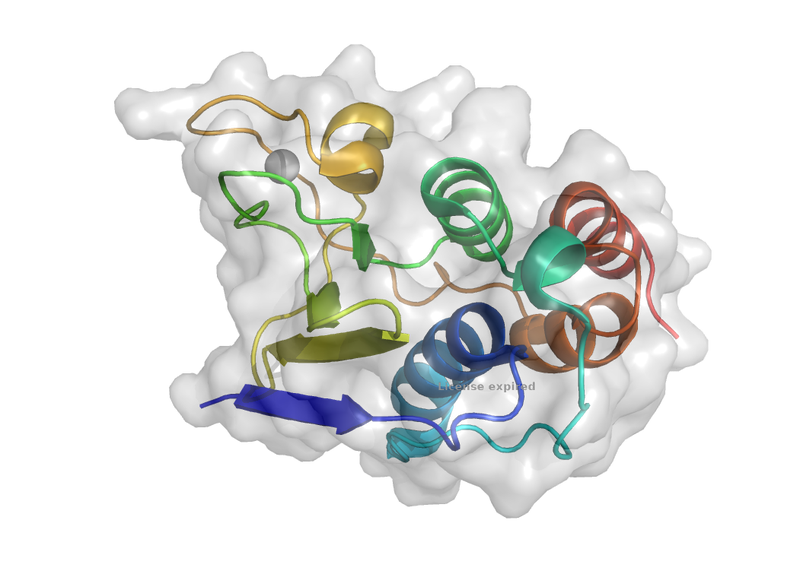
Structure-guided mutations of the PLP2 viral protein highlight the potential of targeting the protease to reduce viral infection.
June 1, 2024
Treatment and prevention of porcine reproductive and respiratory syndrome (PRRSV) imparts a significant cost in commercial agriculture. Positive-sense RNA viruses, including PRRSV and coronaviruses, rely on a very limited number of proteases for viral replication. Targeting the viral protease may lead to a treatment with high potency by targeting the viral replication pathway prior to activation of the viral proteins.
In this work, the authors determined the novel structure of the PLP2 protease with, and without, the substrate ubiquitin. A series of point mutations and homology modelling highlight the critical residues to both recognition of both ubiquitin and catalytic cleavage of the viral polyprotein. Interestingly, the PLP2 protease mutation is particularly impactful in deubiquitination and unique ubiquitin binding pose when compared to structural homologues.