The CMCF has additional equipment available for performing controlled crystal dehydration experiments as well as X-Ray absorption (XAS) experiments, including EXAFS.
XAS
Structures of metalloprotein active sites derived from X-ray crystallography sometimes contain chemical anomalies including elongated bond-lengths or unexpected atomic geometries. Such anomalies occur due to the known limitations inherent to macromolecular crystallography and from lack of adequate restraints (used in structure refinement) for metal sites which can often be without precedent in the small molecule literature. X-ray absorption spectroscopy (XAS) can provide additional information to improve the accuracy of metalloprotein crystal structure solutions. In order to make use of the technique on crystals, virtually no additional sample preparation is required.
Upon request, beamline 08B1-1 can be equipped with the Vortex-ME4 four-element detector which allows for the perfomance of high resolution XAS experiments on crystals by monitoring fluorescence. If you have an extra 2-3 hours to spend on a project, substantial additional information can be obtained above and beyond the crystallographic data. The types of experiments possible include:
- Near-edge/XANES (X-ray Absorption Near Edge Structure); which allows the determination of geometries and oxidation states for atoms of interest.
- EXAFS (Extended X-ray Absorption Fine Structure); allows accurate determination of atomic distances from the absorbing atoms.
- XRF (X-Ray Fluorescence); the excitation scan allows for the identification of metal atoms in samples.
Technical Details & Sample Preparation
The Vortex-ME4 detector is only available on beamline 08B1-1 at this time. Note that these experiments cannot presently be performed in conjunction with the automounter and thus cannot be done by Remote Control. The regular crystallography bases and loops described in the Preparing Samples section can be used so that diffraction data as well as XAS data can be collected on the same sample. When preparing samples, the concentration of the exogenous metal atom of interest should be minimized in the mother liquor and/or cryoprotectant. This means that if the protein has been co-crystallized or the crystals soaked with excess atom of interest, the crystal will normally require washing.
XAS data collection is integrated into MxDC. Please visit the Fluorescence Scans Tab of MxDC for more details.
Data output from XAS data collection with MxDC is contained in a .xdi file, using the standardized XAS interchange specification format which ensures compatibility with mainstream XAS software. For more information, please feel free to contact us. The following publication describes recent advancements in the XAS field at the CMCF:
Cotelesage, J.J.H., Grochulski, P., Pickering, I.J., George, G.N., Fodje, M.N. (2012) X-ray absorption spectroscopy at a protein crystallography facility: the Canadian Light Source beamline 08B1-1. J. Synchrotron Rad. 19(6), 887-891.
Humidity Control
The MAATEL humidity control device (HC1) allows you to dehydrate crystals in a highly-controlled manner and can be installed on either CMCF beamline with prior notice. Dehydration of crystals can in some cases increase the quality and/or resolution of data.
If you have requested that your beamtime be used for humidity control experiments, beamline staff will set up the HC1 for you, normally during the beginning of your first shift. Note that the HC1 nozzle which blows a stream of humidified air onto the crystal sample replaces the cryojet, thus the cryojet will not be in place during this time so it is best to arrange for separate beamtime to collect data once your samples have been prepared using the HC1. These experiments require a substantial amount of patience and careful observation and you need a number of crystals in order to optimize the procedure.
For detailed information on performing these types of experiments, please see: Sanches-Weatherby et al. (2009) Improving diffraction by humidity control: a novel device compatible with X-ray beamlines, Acta Crystallogr. D65, 1237-1246. Further documentation is also available at the beamline.
Basic Procedure
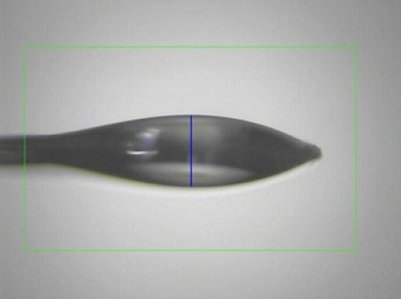
First you will need to find the initial relative humidity in equilibrium with the mother liquor specific to your crystal of interest. This is done using a drop of mother liquor mounted in a standard cryoloop, oriented so that the view is parallel to the plane of the loop. If the humidity level is too low, the size of the drop will decrease whereas if the humidity level is too high, the size of the drop will increase. The size of the drop is measured by a blue vertical line on the MxDC pane which extends from one end of the drop to the other.
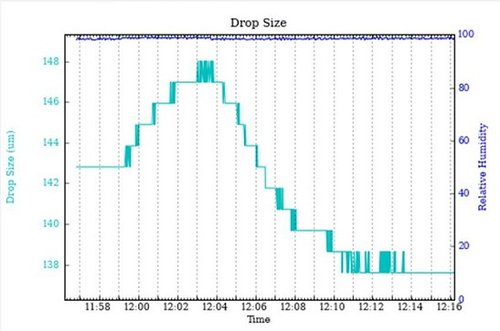
Example: A solution of 70% sucrose was mounted in a cryoloop in the vapour stream of the HC1 with an initial relative humidity setting of 98.5%. The initial increase in drop size indicates a humidity level that is too high. Changing the relative humidity to 98.0% resulted in a decrease in drop size. The drop size remained stable only after the relative humidity was adjusted to 98.2%.
- After you have found the appropriate humidity level, you can mount a crystal sample. It is easiest to mount it onto a mesh-type loop available from MiTeGen.
- You will next dehydrate the crystal in increments and take diffraction images in order to find the best level and rate of dehydration. This is an iterative process that may require many crystals.
- Several samples should be dehydrated using the optimized process and cooled for later data collection. This is normally accomplished by submerging the crystals into liquid nitrogen and saving them.
The HC1 is controlled from MxDC. Please visit the MxDC Samples Tab section for details on controlling the device.